Nex for Medtech
NEX: The Prebuilt Software Foundation for MedTech
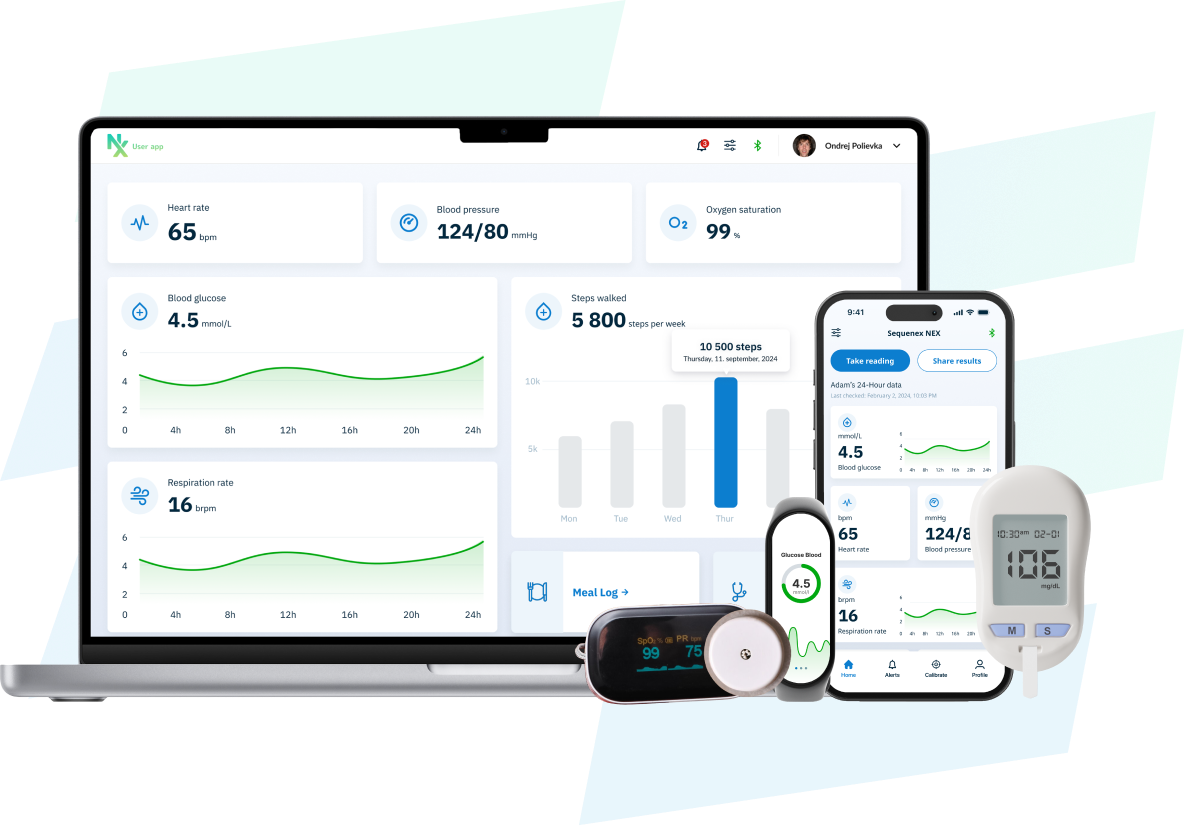
Build Your Device. Not the Software Stack
- Pre-built and structured for regulated devices, NEX delivers the complete mobile, cloud, and device integration stack, including iOS and Android apps, secure backend infrastructure, dashboards, and administrative controls.
Collect, Analyze and Display Real-Time Data in just weeks
Capture CGM, biosensor, wearable, and connected device data through a secure, production-ready mobile and cloud platform. NEX enables real-time ingestion and presentation within a configurable, regulatory-ready software stack. Deploy product software in weeks, not months.
Why Choose NEX?
A purpose-built medtech software foundation — delivered faster, owned by you.
Engineered under formal design controls
NEX is developed within an ISO 13485–certified QMS and governed by formal software design controls aligned with IEC 62304 and ISO 14971.
The platform architecture includes configurable security, role-based access, and audit controls designed for HIPAA safeguards and 21 CFR Part 11.
Software foundation for connected Medical Devices
NEX supports CGMs, biosensors, wearables, and connected medical devices. It delivers the full product software stack — mobile apps, secure cloud, and device integration. Don’t build what’s already been engineered. Deploy NEX and reduce development time by up to 80%.
Built for ownership and control
We build the software. You own the product. NEX is delivered as a complete system and hosted in your environment, giving you full control over your product roadmap, data, architecture, and long-term evolution. NEX adapts to your strategy and workflows, allowing you to move forward without external constraints on how your product grows over time.
Accelerate delivery with a proven medtech partner
By starting with a purpose-built foundation and working with a team experienced in medtech software, companies can avoid years of duplicated effort and reduce development time and cost by up to ~80% compared with building equivalent capabilities from scratch.
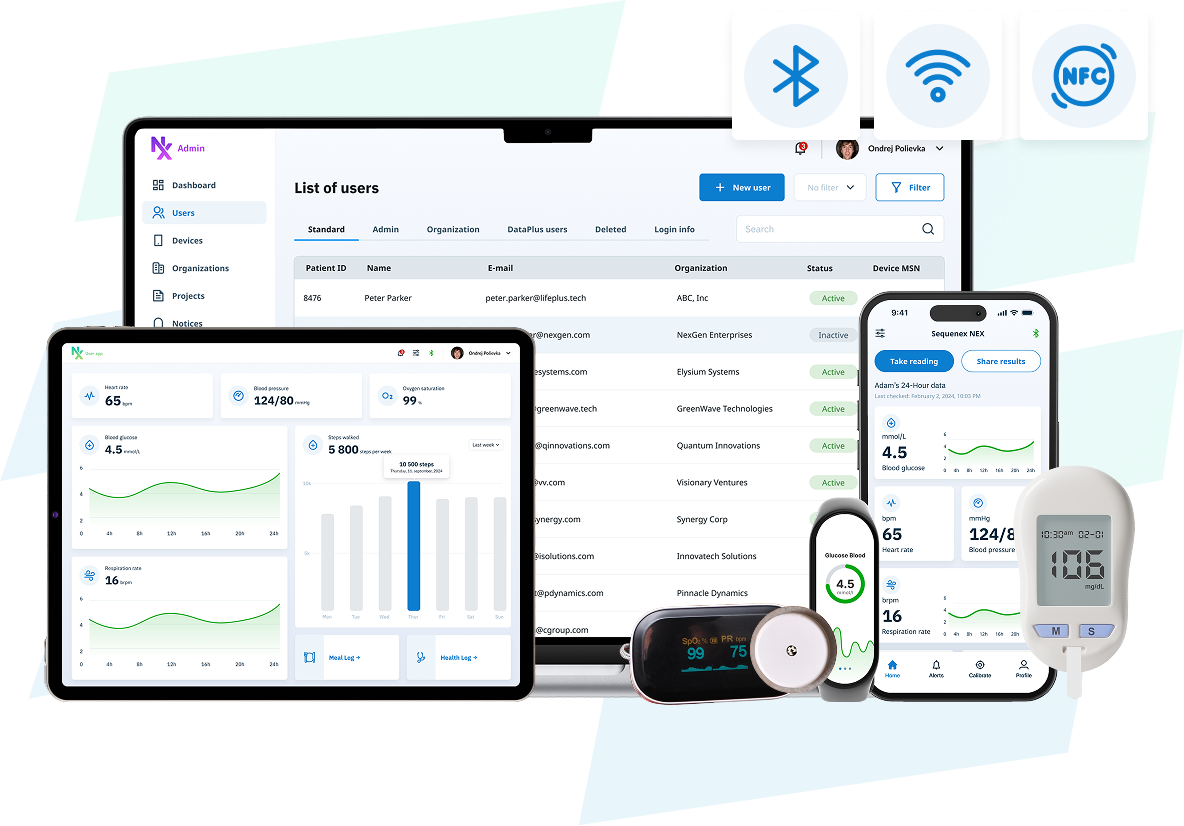
One Foundation - Support for All Development Stages
Prototype
Quickly develop and test prototypes to validate your concepts before full-scale development.MVP
Develop Minimum Viable Products (MVPs) efficiently to test market readiness and demonstrate your product to investors.Clinical
Seamlessly transition to developing clinical applications withbuilt-in compliance features.Commercial
Confidently scale up to full commercial applications using ourreliable prebuilt infrastructure.
Rapid Time to Market
Prebuilt Mobile Apps, Cloud Dashboards, and Admin Portal: Start with fully functional software. Customized and configured to fit your device's unique requirements.Full Service Software and Support
Sequenex fast-tracks software delivery with regulatory expertise, end-to-end software engineering, custom development and prebuilt modules, and ongoing support.
Regulatory Compliance
Show FeaturesCompliant with regulatory requirements and customizable. NEX includes robust “out of the box” features and functionality and allows for a wide variety of customizations. Start your project at 80% done!
Secure & Scalable Cloud Architecture
HIPAA-Compliant Storage, End-to-End Data Encryption, Real Time Data Processing, Comprehensive Cybersecurity Protocols, Data Back Up and Recovery
ISO 13485 Certified QMS
NEX has been engineered, validated and tested under Sequenex’s ISO 13485 Certified Quality Management System, ensuring adherence to the highest regulatory standards in the industry.
Comprehensive Documentation
We provide tailored and required regulatory documentation (DHF, SRS, V&V) to streamline compliance and support your product.
Design Controls
The NEX platform complies with 21 CFR 820.30, IEC 62304 and ISO 14985 ensuring all aspects of the software development process and any modifications are documented and controlled.
Built-In Compliance Features
The NEX platform includes features to maintain continuous compliance with industry standards and regulations, including automated data logging, traceability, and audit trails.
Customization and Flexibility
Configurable UI and Features
Easily customize the platform’s user interface and functionalities to suit your specific needs and branding.Adaptable Architecture
Modify and expand the platform to meet the unique requirements of your device.
Comprehensive Maintenance and Support
Dedicated Engineering
Sequenex operates as an extension of your organization, delivering regulated software expertise across the product lifecycle.
Controlled Change Management
Enhancements and modifications are executed under structured design controls, with impact analysis, testing, and full documentation.
Regulatory Continuity
Preserve design history, risk management, and software lifecycle documentation as your product evolves.
Capital-Efficient Scaling
Expand software capacity as your product grows, without the cost and complexity of building an in-house regulated engineering organization.
Cost and Resource Efficiency
