ISO 13485 Certified QMS
SaMD and Connected Devices Software Experts
Need to Get Your Device Software Up and Running Quickly?
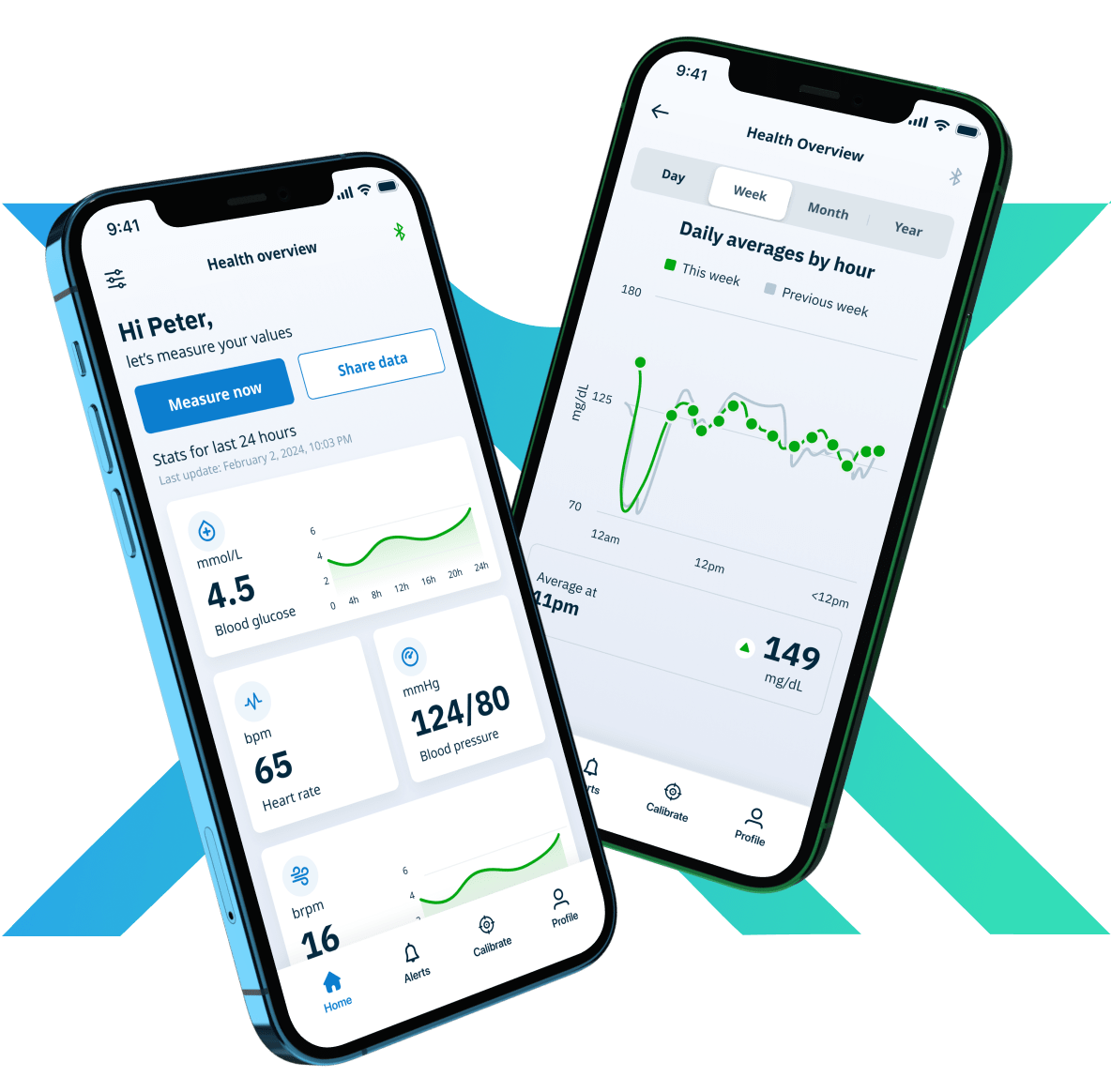
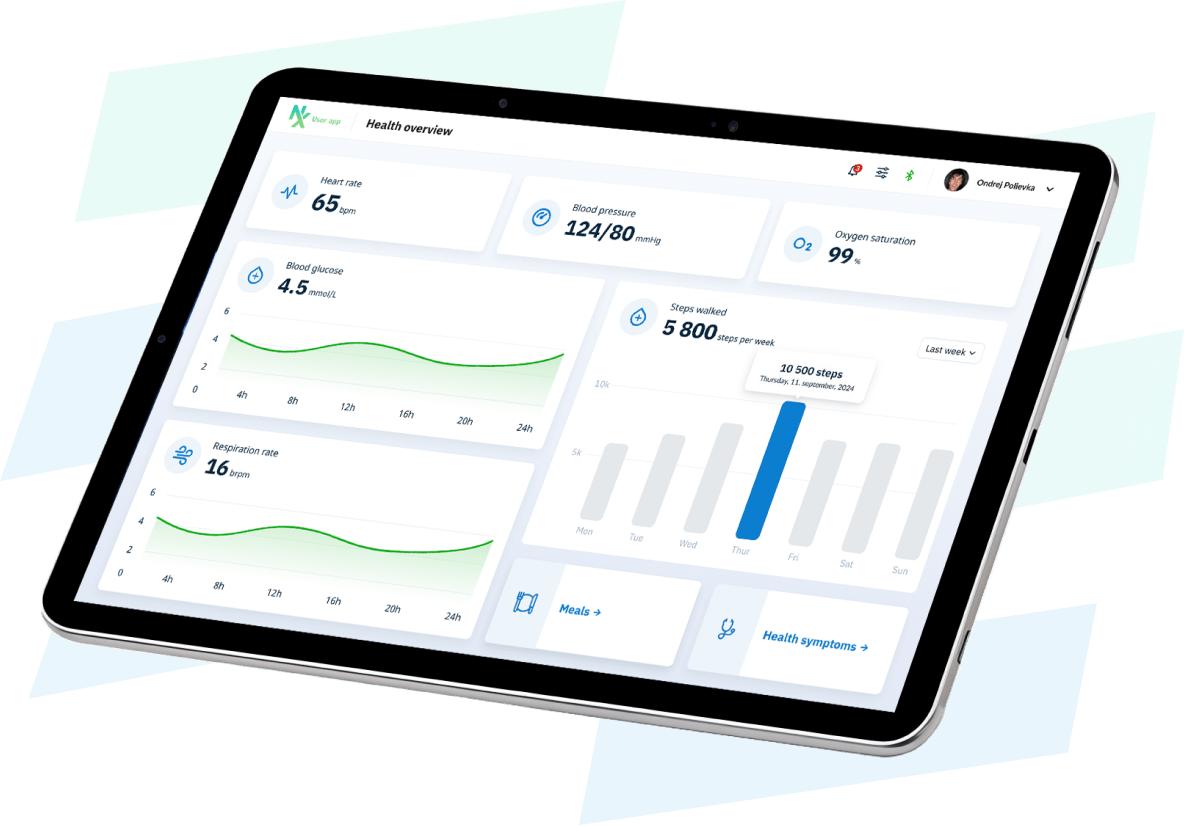
Save more than 80% in time and development costs with our prebuilt and configurable NEX Platform.
Regulatory ready and compliant by design for MedTech. Complete with iOS and Android Companion Apps, Patient and Clinician Dashboards, an Administrator Portal and Secure Cloud.
Our Services
BiosensorsSequenex rapidly develops and delivers comprehensive and compliant Software as a Medical Device (SaMD) solutions and products for the connected device, CGM and biosensor markets.

Testing, QA and V&V
Comprehensive automated and manual test execution across mobile applications, cloud services, and device integrations.

AI and ML Solutions
AI-driven analytics and algorithm development for medical and connected device software, designed for traceability, validation, and regulatory alignment.

Custom Software Development
Full Service. IEC 62304–aligned engineering for medical and digital health software, delivered with full traceability and regulatory documentation.

BLE, NFC, WiFi
Implementation of BLE, NFC, & Wi-Fi communication layers for connected medical devices.

Dedicated MedTech Engineering
Flexible staff augmentation or fully managed teams for SaMD and connected devices.

AI-Enabled Engineering
AI-assisted development, documentation, and testing to accelerate delivery and improve quality while
maintaining controlled, traceable processes aligned with regulated environments.
Cybersecurity and Privacy Solutions
End-to-end security design for mobile apps, cloud systems, and device connectivity layers.
Implements encryption, role-based access, and audit controls.
Custom Firmware Development
Development of custom firmware for biosensor and connected devices to optimize performance, add features, and ensure stable connectivity.
- Conforms with IEC 62304 (SDLC)
- Emphasizes Risk Management throughout Development Lifecycle (ISO 14971)
- Complies with Regulatory Requirements
- Ensures Traceabilty & Accountability
Developed within our ISO 13485–certified QMS, NEX includes a complete software design documentation package prepared for integration into the sponsor’s Design History File.
The Sequenex ISO 13485 Certified QMS
Our ISO 13485 Certified QMS underscores our commitment to excellence in medtech software engineering, ensuring your projects are delivered with unparalleled precision and regulatory compliance.
Our Expertise
We specialize in expediting the delivery of SaMD, adhering to ISO 13485 and IEC 62304 standards. Our expertise spans testing, FDA compliance, and rigorous risk management, ensuring high-quality, safe solutions for healthcare.

SaMD Compliance and Development Processes
Specializing in SaMD, compliant medical device software development, adhering to ISO 13485 and IEC 62304 standards for safety, efficacy, and regulatory compliance.
Testing and Quality Assurance
Expert in rigorous system and subsystem testing — including unit, integration, and acceptance testing. Emphasizes automated testing, comprehensive test case management, and regulatory standards for quality solutions.
Companion Apps Development
Remote Patient Monitoring, Patient Monitoring, Fitness & Wellness, Medication Adherence, Chronic Disease Management, Wearables, Data Analytics & Reporting
Risk Management
Proactively manages risks per ISO 14971 guidelines, including hazard analysis, risk assessments, and mitigation strategies to enhance end-user safety and regulatory compliance.
Diabetes and Biosensor Subject Matter Experts
We specialize in software development for diabetes and biosensor devices with a focus on robust, integration-ready, scalable solutions. CGM, pump and pen expertise.
Quality & Regulatory
We integrate quality and regulatory best practices into every step of the software development process — ensuring your transition from concept to commercial release is seamless.
- Test-driven development
- Automates testing procedures
- Simulates device behavior
- Ensures reliability and performance
- Mobile App Persistence Library: Ensures data integrity
- Graphing Library: Visualizes medical data
- Logging Library (HIPAA compliant): Secure logging
- OIDC/OAuth2 Authentication: Robust authentication mechanisms
- Safeguards sensitive patient information
- Automated Documentation: Automates and generates comprehensive regulatory documentation
Tools and Solutions
We use advanced solutions, tools and processes to expedite medtech and digital health software delivery. We ensure rapid development and strict regulatory compliance for faster market readiness.
Knowledge Hub
SaMD News & Industry Updates.
Explore Knowledge Hub
February 25, 2026
Software Remediation for Medical Devices: The Readiness Gap Most Teams Discover Too Late
Many MedTech teams discover too late that their working software isn’t submission-ready. This article explores common triggers, industry remediation patterns,…
February 6, 2026
How Digital Health Companies Can Simplify Biosensor App Builds and Data Integration
Simplify biosensor app builds and data integration with NEX by Sequenex—a prebuilt, secure platform that helps digital health and wellness…
January 24, 2026
MVPs in MedTech: The Role of Rapid Prototyping in Device Innovation
Rapid prototyping to create functional Minimal Viable Products (MVPs) is a necessary step to accelerate the time to market for…